
The mechanism of action of NaBH4 (upper) and NaBH4Metal salt (bottom)... Download Scientific
Popular answers (1) Pekka Pietikäinen Orion Corporation I preferably use NaBH4/CaCl2 combination for ester reductions. The reaction works selectively in most cases and under mild conditions (RT.
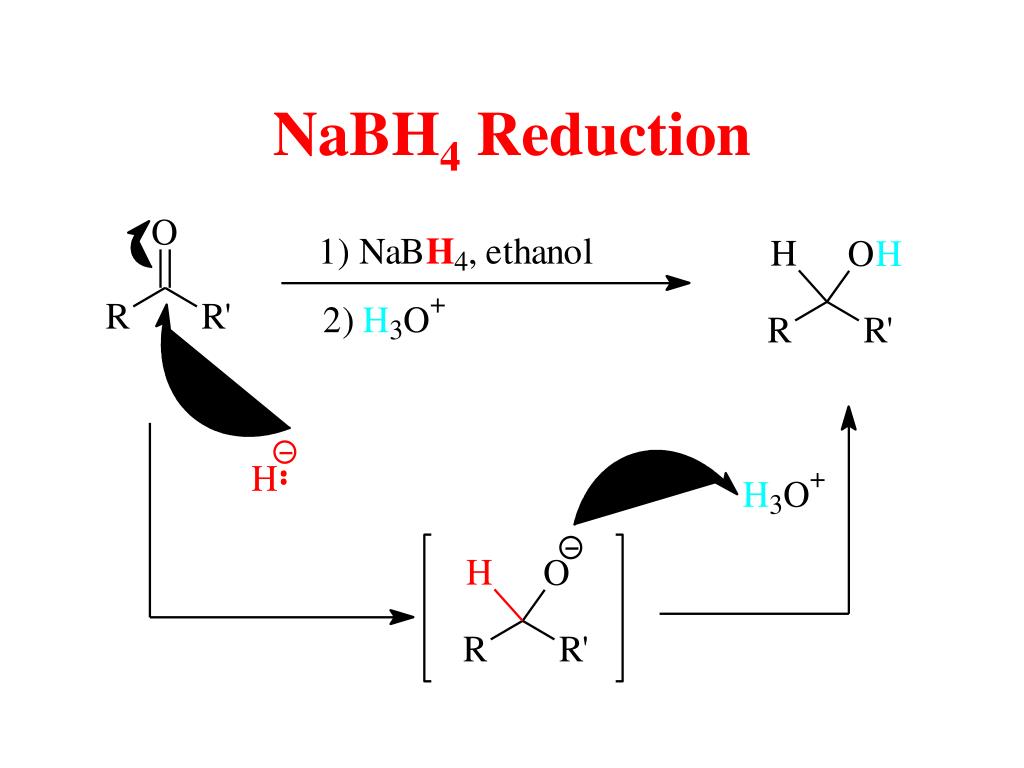
PPT Alcohols PowerPoint Presentation, free download ID4396111
Ester Reduction to a 1 o Alcohol. Esters can be converted to 1 o alcohols using LiAlH 4, while sodium borohydride (NaBH 4) is not a strong enough reducing agent to perform this reaction. The reduction of ethyl benzoate to benzyl alcohol and ethanol is shown as an example.

organic chemistry What is the mechanism for the hydrolysis of the boronalkoxide complex in
In the sodium borohydride reduction the methanol solvent system achieves this hydrolysis automatically. In the lithium aluminium hydride reduction water is usually added in a second step. The lithium, sodium, boron and aluminium end up as soluble inorganic salts at the end of either reaction. Note!
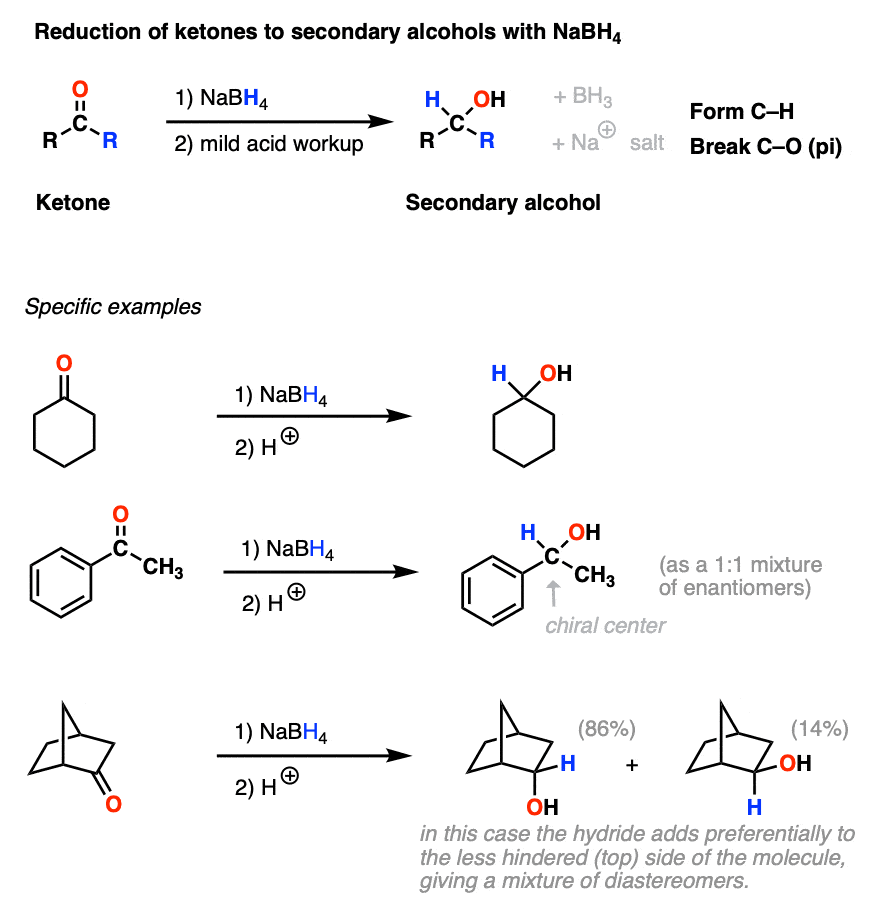
Sodium Borohydride (NaBH4) As A Reagent In Organic Chemistry
COMMON REDUCING AGENTS LiAlH4 LITHIUM ALUMINIUM HYDRIDE (LAH) NaBH4 Non-selective reagent for hydride transfer reductions. Reacts with carboxylic acids, esters, lactones, anhydrides, amides and nitriles, converting them into alcohols and amines. Ketones, aldehydes, epoxides, alkyl halides are also reduced with lithium aluminium hydride.

Efficient and Simple NaBH4 Reduction of Esters at Cationic Micellar Surface Ester Chemical
Prof. Steven Farmer ( Sonoma State University) Esters can be reduced to 1° alcohols using LiAlH4 L i A l H 4 is shared under a CC BY-NC-SA 4.0 license and was authored, remixed, and/or curated by LibreTexts. Back to top. Esters can be converted aldehydes using diisobutylaluminum hydride (DIBAH). General mechanism of ester reactions.

Aldehyde Ketone 17 I Reaction of Ester using LIAlH4 I NaBH4 I Reduction I Reactivity I Prof
Reduction of Esters and Lactones at Room Temperature without Solvent-Induced Loss of Hydride | The Journal of Organic Chemistry RETURN TO ISSUE PREV Article NEXT Stabilization of NaBH4 in Methanol Using a Catalytic Amount of NaOMe. Reduction of Esters and Lactones at Room Temperature without Solvent-Induced Loss of Hydride Prasanth C. P. † ,

LiAlH4 and NaBH4 Carbonyl Reduction Mechanism Chemistry Steps
This large-scale chirality at the interface of self-aggregates was exploited towards asymmetric resolution in ester reduction by NaBH4. An enantiomeric excess of 53% ((R)-2-phenylpropan-1-ol) was found in the case of the n-hexyl ester of 2-phenylpropionic acid as substrate in the aqueous aggregate of N,N'-dihexadecyl-N,N,N',N'-tetramethyl-N,N'-ethanediyldiammonium diquinate.

Proposed mechanism for the reduction of nitroarenes using NaBH4... Download Scientific Diagram
The electron rich and hindered ester, t-amyl-3,4,5-trimethoxybenzoate, also does not reduce under these conditions (with or without LiCl). However, both methyl and isopropyl 3,4,5-trimethoxybenzoate esters were converted into 3,4,5-trimethyoxybenzyl alcohol in good yields in NaBH 4 /diglyme/LiCl at 162°C. These reductions did not occur unless.

Mechanism Study of βketo Ester Reduction using NaBH4/MeOH via Density Functional Theory
Sodium borohydride (NaBH 4) is not a reactive enough hydride agent to reduce esters or carboxylic acids. In fact, NaBH 4 can selectively reduce aldehydes and ketones in the presence of ester functional groups.. The mechanisms for the hydride reduction of esters is analogous to the hydride reduction of carboxylic acids. Nucleophilic acyl.

LiAlH4 and NaBH4 Carbonyl Reduction Mechanism Chemistry Steps
Abstract. An intramolecular hydride delivery process largely contributes during the double reduction of α-keto esters into diols by NaBH 4. In the case of enolic α-keto esters, the first step of the process, the reduction of the keto group, occured exclusively through an 1,2-hydride addition despite the predominance of the tautomeric enolic form.
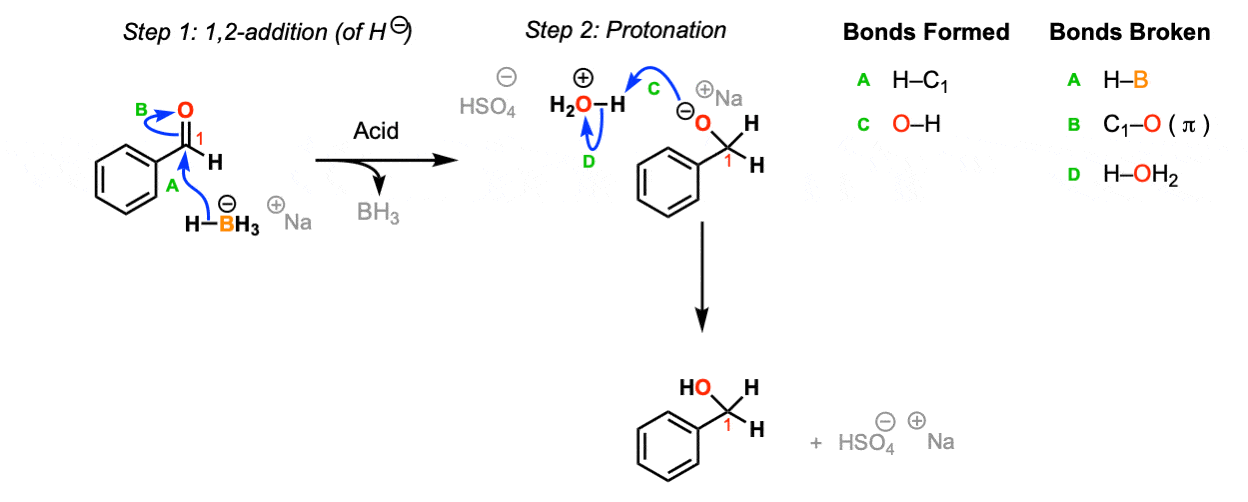
Addition of NaBH4 to aldehydes to give primary alcohols Master Organic Chemistry
Esters (including lactones) and amides are not reduced. As a source of hydride ion, NaBH will also act as a strong base, deprotonating water, alcohols, and carboxylic acids. also sees use in the reduction of organomercury bonds after oxymercuration reactions. 1. Sodium Borohydride (NaBH

Sodium Borohydride In Organic Chemistry
In the sodium borohydride reduction the methanol solvent system achieves this hydrolysis automatically. In the lithium aluminium hydride reduction water is usually added in a second step. The lithium, sodium, boron and aluminium end up as soluble inorganic salts at the end of either reaction.. Reduction of carboxylic acids and esters.

Stabilization of NaBH4 in Methanol Using a Catalytic Amount of NaOMe. Reduction of Esters and
This large-scale chirality at the interface of self-aggregates was exploited towards asymmetric resolution in ester reduction by NaBH 4. An enantiomeric excess of 53 % (( R )-2-phenylpropan-1-ol) was found in the case of the n -hexyl ester of 2-phenylpropionic acid as substrate in the aqueous aggregate of N , N ′-dihexadecyl- N , N , N ′, N ′-tetramethyl- N , N ′-ethanediyldiammonium.

Scheme 1. Sodium borohydride (NaBH4) and diisobutylaluminum hydride... Download Scientific Diagram
Ester to Alcohol (NaBH 4) Examples: Example 1 To a suspension of the SM (500 mg, 0.99 mmol) in MeOH (50 mL) at 0 C was added NaBH4 (113 mg, 2.96 mmol). The reaction mixture was stirred at RT for 2 h. After concentration, the residue was diluted with H2O (100 mL) and extracted with EtOAc (3 x 30 mL).
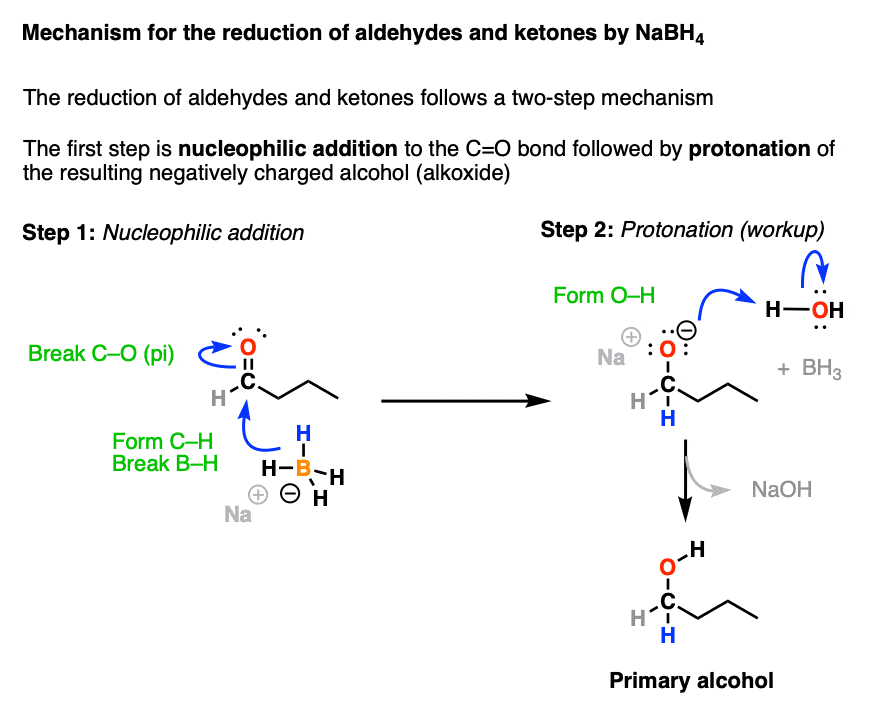
Sodium Borohydride (NaBH4) As A Reagent In Organic Chemistry
Sodium borohydride, also known as sodium tetrahydridoborate and sodium tetrahydroborate, [5] is an inorganic compound with the formula Na B H 4 (sometimes written as Na [BH4] ). It is a white crystalline solid, usually encountered as an aqueous basic solution.
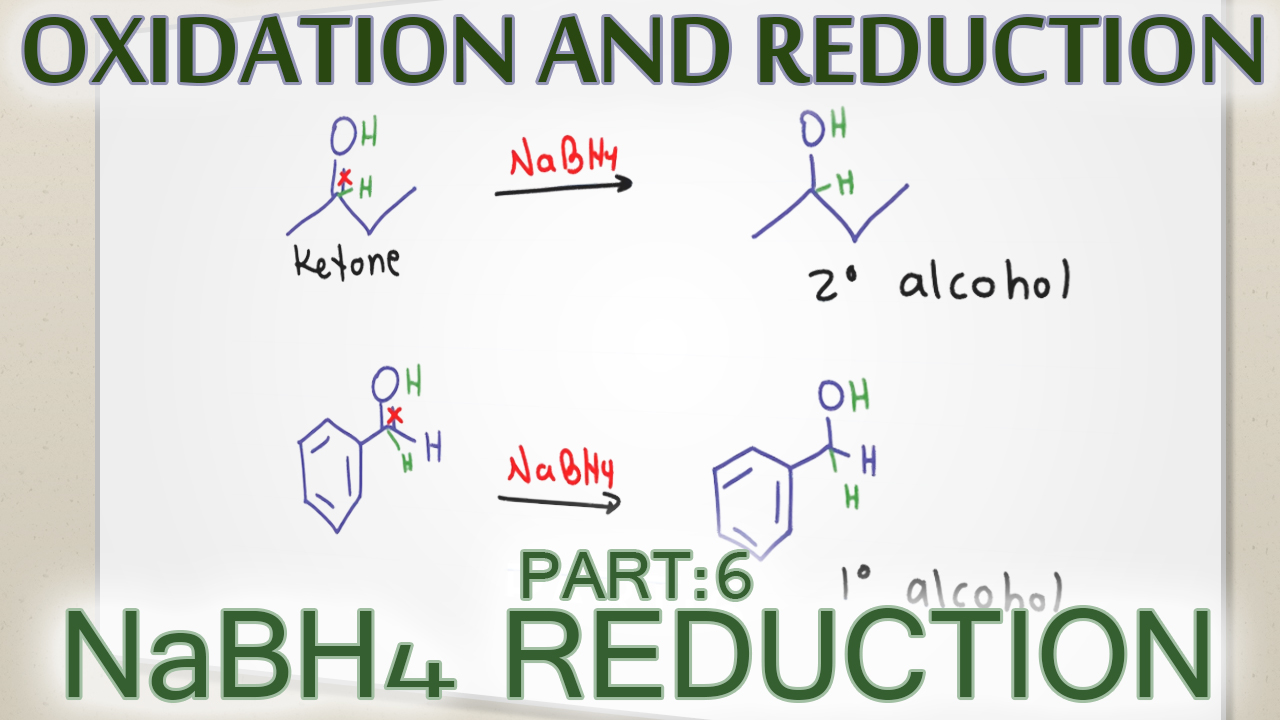
Sodium Borohydride Carbonyl Reduction Reaction and Mechanism
Rapid reaction of NaBH 4 with MeOH precludes its use as a solvent for large-scale ester reductions. We have now learned that a catalytic amount of NaOMe (5 mol %) stabilizes NaBH 4 solutions in methanol at 25 °C and permits the use of these solutions for the reduction of esters to alcohols. The generality of this reduction method was demonstrated using 22 esters including esters of naturally.